Keep Taking Steps
in the Right Direction
to Help Treat Your
Type 2 Diabetes
and High
Cholesterol
Photos depict models, not actual patients or healthcare professionals.
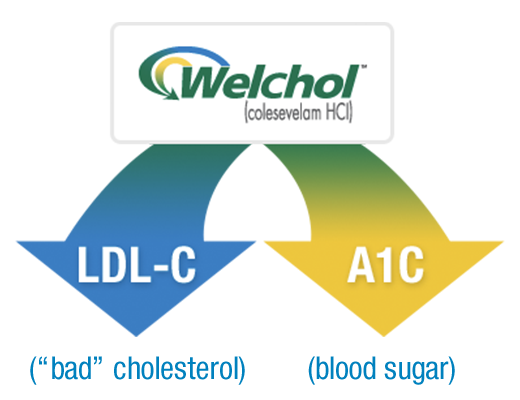
Welchol, along with diet and exercise, is the only FDA-approved medicine with a single active ingredient proven to lower both high blood sugar (A1C) and LDL-C or “bad” cholesterol in adults with type 2 diabetes and high cholesterol.
Benefits of Welchol

WELCHOL is not for everyone. Please see Important Safety Information below.
GET INFORMEDTaking Welchol

 Phenylketonurics: Welchol for Oral Suspension contains 27 mg phenylalanine per 3.75 gram dose.
Phenylketonurics: Welchol for Oral Suspension contains 27 mg phenylalanine per 3.75 gram dose.Phenylalanine can be harmful to patients with phenylketonuria (PKU).
Get Welchol Savings
Print your Welchol Savings Card and start saving today!‡
Based on a $0 co-pay for a 90-day supply or a $10 co-pay for a 30-day supply.‡Restrictions apply based on eligibility.


Based on a $0 co-pay for a 90-day supply or a $10 co-pay for a 30-day supply.‡Restrictions apply based on eligibility.
What is Welchol (colesevelam HCI)?
Welchol, along with diet and exercise, lowers LDL or “bad” cholesterol.
Welchol lowers LDL cholesterol in boys, and in girls who have had a menstrual period, ages 10 to 17 years, with a condition known as heterozygous familial hypercholesterolemia (a genetic disorder that causes high cholesterol).
Welchol, along with diet and exercise, also lowers blood sugar levels in adult patients with type 2 diabetes mellitus.
Welchol should not be used to treat type 1 diabetes or diabetic ketoacidosis.
Welchol has not been shown to prevent heart disease or heart attacks.
Welchol has not been studied with all anti-diabetes medications.
Welchol has not been studied in all types of hypercholesterolemia.
Welchol has not been studied in children younger than 10 years old or in girls who have not had a menstrual period.
‡Savings Card Offer: Eligibility Criteria and Terms & Conditions
To the Patient: You must present this card to the pharmacist along with your WELCHOL® (colesevelam HCl) prescription to participate in the program. For patients with commercial insurance, savings per prescription of WELCHOL will apply after the following out‐of‐pocket expenses are met: $10 per prescription for a 30‐day supply of WELCHOL or $0 per prescription for a 90‐day supply of WELCHOL. Offer may not be combined with any other program offer or discount for WELCHOL. Savings for WELCHOL are subject to a maximum benefit of $150 per 30‐day prescription or $450 per 90‐day prescription. If you have questions regarding your eligibility or benefits, or wish to discontinue participation, call (877) 264 2440 (8 AM – 8 PM ET, Monday‐Friday). When you use this card, you are certifying that you understand the program rules, regulations, and terms and conditions. You are not eligible if you are enrolled in any state or federal health care program, including, but not limited to, Medicare Part D or Medicaid, VA, DOD, or TRICARE/CHAMPUS; or where taxed, restricted, or prohibited by law; or if you do not otherwise comply with the terms of this card. Further, you agree to discontinue using the card if you enroll in any state or federal health care program during the program period. Offer valid in US and Puerto Rico only.
To the Pharmacist: When you use this card, you are certifying that the patient is not enrolled in any federal, state, or other governmental programs for this prescription.
- Submit transaction to McKesson Corporation, using BIN #610524.
- If primary coverage exists, input card information as secondary coverage and transmit using the COB segment of NCPDP transaction. Applicable discounts will be displayed in the transaction response.
- Acceptance of this card is subject to LoyaltyScript® program Terms and Conditions posted at www.mckesson.com/mprstnc.
- Patient not eligible if enrolled in any state or federal health care program, including, but not limited to, Medicare Part D or Medicaid, VA, DOD, or TRICARE/CHAMPUS, or where taxed, restricted, or prohibited by law. Offer valid in US and Puerto Rico only.
- The LoyaltyScript® card is not valid for use with any other prescription drug discount or cash cards for WELCHOL. Claims submitted utilizing the program are subject to audit or validation.
- LoyaltyScript® is not an insurance card.
Cosette Pharmaceuticals, Inc., reserves the right to rescind, revoke, or amend this program, at any time, without notice.
Trademarks not owned by Cosette Pharmaceuticals, Inc., are property of their respective owners.
To contact us with questions or concerns about a Cosette Pharmaceuticals product,
please call us: 1-800-922-1038
WELCHOL is a registered trademark of Cosette Pharmaceuticals, Inc.
© 2022 Cosette Pharmaceuticals, Inc. CP-US-WC-001 06/22


